Given the following and that R = 8.314 J/K.mol,determine K at 298K for the reaction,
AgCl(s) Ag+(aq) + Cl-(aq) 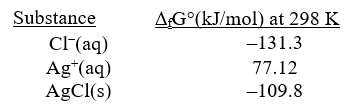
Definitions:
Positive Emphasis
Highlighting the beneficial or successful aspects in a situation or analysis to encourage a favorable view or outcome.
Techniques
Specific methods or ways of doing something, often involving a skill or expertise in a particular area.
Nonsexist Job Title
Professional titles designed to be gender-neutral, avoiding discrimination and promoting equality in the workplace.
Flight Attendant
An airline employee responsible for ensuring the safety, comfort, and care of passengers during flights.
Q1: Which of the following units is
Q23: The period 6 transition metals have greater
Q26: What is the name given to a
Q32: How many mechanistic steps are depicted by
Q38: Identify the following orbital. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="Identify
Q43: The pH of a solution at
Q61: A 25.0 mL sample of 0.10
Q61: What is the conjugate base of [Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>(aq)?<br>A)
Q67: Sodium chloride crystallizes in a(n)_ cubic unit
Q76: What is the OH<sup>-</sup> concentration in