The standard free energy change associated with the dissolution of ammonium nitrate in water is -6.73 kJ/mol at 298.15 K.
NH4NO3(s) 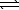 NH4NO3(aq)
NH4NO3(aq)
What is the equilibrium constant for the reaction? (R = 8.314 J/K.mol)
Definitions:
Coolie Trade
The historical trade of laborers from Asia, particularly China and India, under contracts that were often exploitative, to work in various parts of the world.
Indentured Laborers
Individuals who worked for a specified period in exchange for passage to the New World, often under harsh conditions.
Chinese Laborers
Workers from China, often who were recruited in the 19th and early 20th centuries to perform labor-intensive tasks, notably on the Transcontinental Railroad and in mining operations.
Sex Workers
Individuals who engage in sexual activities in exchange for money or goods, often within a structured industry.
Q1: What is the oxidation state of each
Q19: Concentrated sodium hydroxide is 19.4 M and
Q19: Nitrogen dioxide reacts with carbon monoxide
Q21: A salt with a 1:1 ratio of
Q34: What is the general formula for an
Q35: Which of the following statements concerning
Q48: What is the K<sub>c</sub> expression for the
Q62: Which of the following reactions would
Q69: Write the expression for K<sub>p</sub> for the
Q80: The primary source material for the commercial