Calculate 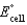 for the electrochemical cell below,
for the electrochemical cell below,
Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s)
Given the following reduction half-reactions.
Pb2+(aq) + 2 e- Pb(s) E = -0.126 V
PbCl2(s) + 2 e- Pb(s) + 2 Cl-(aq) E = -0.267 V
Fe3+(aq) + e- Fe2+(aq) E = +0.771 V
Fe2+(aq) + e- Fe(s) E = -0.44 V
Definitions:
Terms of Trade
The ratio between a country's export prices and its import prices.
Imported Product
Assets or services sourced from outside a country and brought in for the objective of sales.
Domestic Product
The total value of all goods and services produced within a country's borders in a specified time period.
Exchange Rate
The price of one currency in terms of another, determining how much foreign currency can be exchanged for the domestic currency.
Q17: Aluminum is resistant to corrosion because<br>A) the
Q17: What is the pH of a
Q19: Calculate E°<sub>cell</sub> for the cell for
Q20: What two of the following elements or
Q23: Calculate the standard entropy change for
Q32: Which of the following equilibria would not
Q42: The K<sub>a</sub> for the monoprotic acid
Q44: Which statement concerning relative rates of
Q75: What is the H<sub>3</sub>O<sup>+</sup> concentration of
Q92: Which of the following reactions is