The following has a potential of 0.92 V: 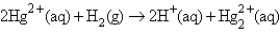 If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then E for the half-reaction
If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then E for the half-reaction 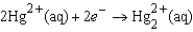
Would be
Definitions:
Validity
The extent to which a test or instrument measures what it is supposed to measure.
Predictive
Relating to the ability to foresee or anticipate future events or outcomes based on current data or trends.
Standardization
The process of developing and implementing technical standards to ensure consistency and compatibility between processes and products.
Predictive Validity
The extent to which a score on a scale or test predicts future performance on a related measure.
Q16: Which functional group does <span
Q28: What is the hydronium-ion concentration of
Q28: Which of the following is not a
Q45: What is the correct name for NO<sub>3</sub><sup>-</sup>?<br>A)
Q48: A 50.00-mL solution of 0.0350 M
Q49: Which of the following do
Q56: All of the following are colloidal dispersions
Q60: Which of the following statements concerning
Q60: Two important biological buffer systems control pH
Q84: What color change is exhibited by phenolphthalein