What is the value of the reaction quotient,Q,for the voltaic cell constructed from the following two half-reactions when the Zn2+ concentration is 0.0120 M and the Ag+ concentration is 1.25 M? 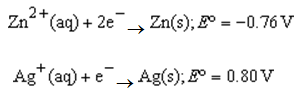
Definitions:
Contractual Liability
Legal obligations arising from contracts entered into by an entity or individual.
Warranty Liability
The legal obligation of a seller to ensure the goods or services sold meet certain quality and performance standards.
Dishonor
The refusal or inability to pay a bill of exchange or fulfill a financial obligation when due.
Presentment
The formal presentation of a financial instrument, such as a check, for payment.
Q7: Concentrated nitric acid reacts with copper to
Q7: Which of the following elements undergoes nuclear
Q22: Which of the following nuclides has the
Q40: Ammonium perchlorate can decompose violently according
Q49: Identify the product(s)of the hydrogenation of cis-2-hexene.<br>A)
Q62: Which element from the first transition series
Q69: The decomposition of phosphine,PH<sub>3</sub>,follows first-order kinetics.<br>4
Q77: When a nucleus undergoes radioactive decay,its new
Q78: If K<sub>c</sub> = 0.152 for A<sub>2</sub> +
Q79: What is the correct formula for sodium