Calculate the equilibrium constant for the following reaction at 25 C,
2 IO3-(aq) + 5 Hg( 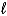 ) + 12 H+(aq) I2(s) + 5 Hg2+(aq) + 6 H2O(
) + 12 H+(aq) I2(s) + 5 Hg2+(aq) + 6 H2O( 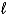 )
)
Given the following thermodynamic information.
IO3-(aq) + 6 H+(aq) + 5 e- I2(s) + 3 H2O( 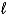 ) E = +1.20 V
) E = +1.20 V
Hg2+(aq) + 2 e- Hg( 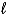 ) E = +0.86 V
) E = +0.86 V
Definitions:
Completing The Square
A method used to solve quadratic equations by adding a constant term to both sides to form a perfect square trinomial.
Compounded Annually
The process of calculating interest on both the initial principle and the accumulated interest of previous periods once per year.
Interest Rate
The percentage of a sum of money charged for its use, typically expressed as an annual percentage.
Quadratic Form
An expression in algebra where the highest power of a variable is squared, typically in the format ax^2 + bx + c.
Q13: Write balanced reduction and oxidation half-reactions for
Q18: An aqueous solution with a pH of
Q20: What two of the following elements or
Q20: An aqueous mixture of phenol and ammonia
Q26: Which ionic compound forms a pH-neutral
Q32: Which of the following statements is/are
Q41: Classification of a compound as a lipid
Q46: Oxides of the alkaline earth family form
Q57: A second-order reaction starts with an
Q71: Write a balanced chemical equation for