Calculate 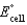 for the electrochemical cell below,
for the electrochemical cell below,
Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s)
Given the following reduction half-reactions.
Pb2+(aq) + 2 e- Pb(s) E = -0.126 V
PbCl2(s) + 2 e- Pb(s) + 2 Cl-(aq) E = -0.267 V
Fe3+(aq) + e- Fe2+(aq) E = +0.771 V
Fe2+(aq) + e- Fe(s) E = -0.44 V
Definitions:
Emissions Tax
A tax on the production, sale, or use of goods that produce pollutants, intended to reduce the harm they cause to the environment.
Marginal Benefit
The added gain achieved by consuming an additional unit of a product or service.
Marginal Benefit
The enhanced pleasure or value a person gains upon consuming one more unit of a particular good or service.
Emissions Tax
A tax imposed on companies for the pollution they produce, intended to motivate them to reduce pollutants and adopt cleaner processes.
Q2: The use of electrical energy to produce
Q20: Which ion occurs in the highest concentration
Q31: What is the concentration of silver(I)ion
Q43: Which relationship correctly compares the rates
Q45: Elemental sodium is commercially produced by electrolysis
Q51: The concentration of magnesium carbonate in a
Q52: What is a correct cell notation
Q67: What class of compounds is responsible for
Q77: The active ingredients in "strike anywhere" matches
Q90: H<sub>3</sub>PO<sub>3</sub> is a diprotic weak acid.What is