If rG for the following reaction is -22.2 kJ/mol-rxn,calculate 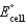 .
.
Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) Cu(s) + 2 AgCl(s)
Definitions:
Expenses
The costs incurred in the process of earning revenue, categorized as operating or non-operating depending on their nature.
Product Costs
Costs that are incurred to acquire or manufacture a product and include direct materials, direct labor, and manufacturing overhead.
General Costs
Broadly refers to the overhead and administrative expenses that are not directly tied to any specific department or product.
Indirect Labor
Labor costs not directly associated with the production of goods, such as maintenance and cleaning staff salaries.
Q3: A 2.5 L flask is filled with
Q18: When a weak base is titrated with
Q22: Which of the following nuclides has the
Q25: What is the minimum mass of
Q33: If a nucleus undergoes positron particle emission<br>A)
Q38: Which of the following statements concerning cell
Q48: Which of the following statements is
Q61: Which of the following is the general
Q61: What is the conjugate base of [Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>(aq)?<br>A)
Q79: Consider the titration of 300.0 mL