What quantity,in moles,of oxygen is consumed when 369.3 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 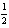 O2(g) H2O( ) ; rH° = -285.8 kJ/mol-rxn
O2(g) H2O( ) ; rH° = -285.8 kJ/mol-rxn
Definitions:
Industry
A sector of the economy characterized by a specific type of activity or product, such as manufacturing, technology, or services.
Firms
Business organizations that produce goods or provide services with the aim of making a profit.
Resources
Assets, materials, and inputs needed for the production of goods and services, including natural, human, and capital resources.
Market System
A market-based economic system where decisions about investment, production, and the allocation of goods are driven by the dynamics of supply and demand, with goods and services' prices being freely determined.
Q2: If a chemical reaction occurs in a
Q11: In a suit against Owen, Phil obtains
Q16: Which functional group does <span
Q22: If an octahedral iron(II)complex is paramagnetic,which
Q30: Write a balanced chemical equation for
Q33: If the reaction quotient,Q,is equal to K
Q56: A plaintiff is a person against whom
Q62: Which three steps,placed in the proper order,are
Q62: The reaction A <span class="ql-formula"
Q65: Based on the following data,what is