In an octahedral complex,electrons in 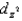 and
and 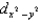
Orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals
Definitions:
Authorized
Officially given permission or power to do something or to act in a certain capacity.
Implied Authority
Authority that is created not by an explicit oral or written agreement but by implication or inference. In agency law, implied authority of the agent can arise from custom, from the position the agent occupies, or from being reasonably necessary to carry out express authority.
Express Authority
Authority expressly given by one party to another. In agency law, an agent has express authority to act for a principal if both parties agree, orally or in writing, that an agency relationship exists in which the agent has the power (authority) to act in the place of, and on behalf of, the principal.
Exclusive Territory
A designated area or market granted to an individual or entity with the exclusive right to sell or distribute goods or services within it.
Q5: The iron produced in a blast furnace
Q27: Discount Mart, Inc., files a suit in
Q29: In Export Co.v.Imports, Inc., there is no
Q36: Complete the following fission reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q43: What is the formula for the pentaamminehydroxoiron(III)ion?<br>A)
Q45: What is the copper(II)-ion concentration at 25°C
Q61: What is the nuclear binding energy
Q62: Silicon is purified in a process called
Q69: If 0.50 L of a buffer containing
Q80: The primary source material for the commercial