Calculate the mass defect for an atom of 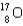 whose isotope mass is 16.9991 g/mol.mass of proton (
whose isotope mass is 16.9991 g/mol.mass of proton ( 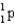 )
)
1) 00783 g/mol.mass of neutron ( 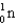 )
)
1) 00867 g/mol.
Definitions:
Standard Errors
The standard deviation of the sampling distribution of a statistic, typically the mean.
Control Chart
A control chart is a statistical tool used to monitor, control, and improve process performance over time by identifying sources of variation.
Process Variability
The natural or inherent variations in a process, reflecting the differences in output or results when the process is repeated under the same conditions.
S Chart
A type of control chart used for monitoring the variability of a process over time, particularly when sample sizes are constant.
Q2: Fleet Feet, an online travel company, buys
Q7: A federal law that conflicts with the
Q13: The iron obtained from the blast furnace
Q19: Julia is a U.S.citizen.She establishes a website
Q21: Setting realistic workplace goals can increase the
Q23: If the meaning of a statute's language
Q24: A disaccharide is formed when two monosaccharides
Q44: Americans with a Better Cause (ABC), a
Q55: Which of the following statements concerning radiation
Q59: The becquerel is an SI unit