Calculate the mass defect for an atom of 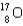 whose isotope mass is 16.9991 g/mol.mass of proton (
whose isotope mass is 16.9991 g/mol.mass of proton ( 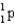 )
)
1) 00783 g/mol.mass of neutron ( 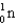 )
)
1) 00867 g/mol.
Definitions:
Elizabeth Loftus
A cognitive psychologist known for her extensive research on the malleability of human memory and the misinformation effect.
Lost At The Mall
A technique used in psychological experiments to implant false memories by suggesting to participants they got lost in a mall as a child.
Sketchy Memories
Memories that are vague, incomplete, or imprecise, often causing uncertainty or suspicion.
Korsakoff's Syndrome
A chronic memory disorder caused by severe deficiency of thiamine, most commonly caused by alcohol misuse.
Q6: Moby, a resident of New Jersey, has
Q19: The types of product defects that have
Q37: Jim operates Jim's Fruits & Vegetables, a
Q42: Which of the following is not a
Q44: An Internet service provider cannot be held
Q45: Which of the following represents the linkage
Q56: Administrative agencies cannot make legislative rules, or
Q78: Which of the following salts would
Q80: The primary source material for the commercial
Q87: Reduction of a ketone produces a(n)_.<br>A) alkane<br>B)