Which of the following compounds have the highest boiling point? 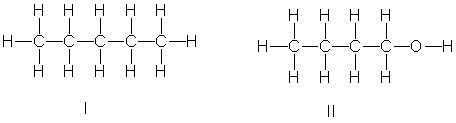
Definitions:
Boiling Point
The temperature at which a liquid turns to vapor, specific to each substance under given pressure conditions.
Diethyl Ether
A highly volatile and flammable ether used as a solvent and formerly as an anesthetic.
Butan-1-ol
A primary alcohol with the formula C4H9OH, where the hydroxyl group is attached to the first carbon of a four-carbon chain.
Ethers
Ethers are a class of organic compounds characterized by an oxygen atom connected to two alkyl or aryl groups.
Q6: Draw all lone pairs of electrons for
Q15: Which of the following has been suggested
Q19: How many pi bonds are present in
Q21: Jumping and screaming in response to an
Q48: According to the two-factor theory of avoidance
Q63: Draw an energy diagram for the conformational
Q82: What is the percentage of the R
Q86: Which of the following is true regarding
Q90: Imagine you are attending a very fancy
Q116: Summarize the rough order in which each