Provide the correct condensed structure for the following compound. 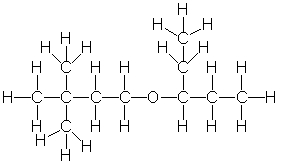
Definitions:
Chemicals
Substances with a distinct molecular composition that are used or produced in processes involving chemical reactions.
pH
A measure of the acidity or alkalinity of a solution, defined by the negative logarithm of the hydrogen ion concentration.
Buffer System
A chemical system that maintains the pH of a solution by adjusting the proportion of acid and base.
Hemoglobin Buffer
A system in red blood cells that helps maintain pH balance in the blood by binding to or releasing hydrogen ions.
Q4: Describe the four basic processes involved in
Q14: Which of the following is a wedge
Q25: A fetus is sufficiently developed to start
Q34: What is the percentage of the S
Q41: The bonds indicated by the arrow in
Q54: What is the leveling effect?
Q57: Draw the enantiomer of the following compound.
Q104: Which of the following can exist as
Q112: Interaction of the following two atomic orbitals
Q128: Draw a bond-line structure for each constitutional