Using a Frost circle, draw the molecular orbital energy diagram for the cyclopropenyl anion and predict if it is aromatic. 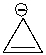
Definitions:
Conjugated Pi System
A molecular structure where alternating single and double bonds allow electrons to be more delocalized and contribute to the molecule's stability.
LUMO
The Lowest Unoccupied Molecular Orbital is the orbital with the lowest energy level capable of accepting an electron.
1,3-Butadiene
An organic compound that is a simple conjugated diene with the formula C4H6, known for its role in the production of synthetic rubber.
Allylic Carbons
Atoms of carbon that are positioned next to a carbon-carbon double bond in a molecule, often exhibiting unique reactivity due to their position.
Q4: Which diene and dienophile would react to
Q16: Predict the possible major products for the
Q16: Predict the product for the following reaction
Q28: Arrange the following electromagnetic radiation in decreasing
Q34: Determine the multiplicity and predict the chemical
Q82: Predict the product for the following reaction.
Q88: Identify the pericyclic reaction in which two
Q99: How many nuclear spin states are possible
Q104: Provide the structure of the major product(s)
Q106: Which of the following is true about