Both pyridine and pyrrole are nitrogen containing aromatic heterocyclic compounds. When treated with HCl, only pyridine forms the hydrochloride salt, whereas pyrrole is unreactive. Provide an explanation for this observed reactivity. 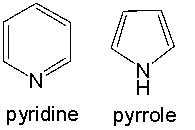
Definitions:
Boiling Point
The boiling point is the temperature at which a substance transitions from a liquid to a gas (vaporizes) at a given pressure, distinctive for each substance.
Molecular Weight
The sum of the atomic weights of all atoms in a molecule, indicating the size of the molecule.
Diabetics
Individuals suffering from diabetes, a condition characterized by high blood sugar levels due to insulin production or response issues.
Insulin
A hormone produced by the pancreas that regulates blood sugar levels by facilitating the uptake of glucose into cells.
Q21: A compound with molecular formula C<sub>5</sub>H<sub>12</sub>O<sub>2</sub> displays
Q29: Which diene and dienophile would react to
Q33: Which of the following compounds will display
Q38: Propose a stepwise synthesis for the following
Q38: Which of the following is not a
Q75: How would you use <sup>1</sup>H NMR spectroscopy
Q94: Identify the reagents used to carry out
Q122: Which of the indicated protons in the
Q125: A compound, with molecular formula C<sub>11</sub>H<sub>14</sub>O, displays
Q132: Which of the following reagent(s) can be