Classify the following compounds as aromatic, antiaromatic, or nonaromatic. Explain your choice. 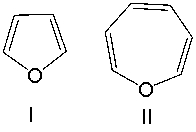
Definitions:
C-H Bond(s)
The chemical bond between a carbon atom and a hydrogen atom, fundamental to the structure of all organic molecules.
Electronegativity Difference
A measure of the ability of an atom in a molecule to attract electrons to itself compared to another atom.
H-Se Bonds
Bonds between hydrogen and selenium atoms, often found in organoselenium compounds and playing roles in some enzymes.
Electronegativity Difference
The measure of the tendency of an atom to attract a bonding pair of electrons towards itself when bonded with another atom.
Q36: Which of the indicated protons in the
Q40: Provide the reagents necessary to carry out
Q50: Predict the product for the following reaction.
Q50: Predict the possible products for the following
Q53: A compound with molecular formula C<sub>9</sub>H<sub>10</sub>O<sub>2</sub>
Q60: Which of the following compounds is consistent
Q93: How many signals would you expect in
Q105: Which one of the following reactions is
Q111: Identify the color of a compound that
Q134: For which of the following compounds will