When applying VSEPR theory to determine the geometry about a central atom,it is important to count the total number of bonded and nonbonded electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many bonded electron groups must be considered for each of these central atoms? 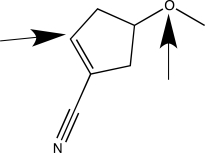
Definitions:
Physical Symptoms
Bodily signs of a condition, disease, or injury, observable through physical examination or patient reporting.
Total Fertility Rate
The average number of children per lifetime birthed by a nation’s women.
Population Constant
A condition where the size of a population remains unchanged over time due to balanced birth and death rates.
Productive Resources
Factors used in the production of goods and services, which include land, labor, capital, and entrepreneurship.
Q4: Draw the product(s)of the following E1 reaction.
Q23: What is the maximum possible number of
Q32: Draw a stereoisomer that has the same
Q41: Draw a detailed mechanism for the following
Q42: Draw all possible resonance forms for anisole
Q62: A compound with a superimposable mirror image
Q68: What is the IUPAC name for the
Q70: How are partners' investments in a partnership
Q120: Partners can invest both assets and liabilities
Q140: The _ principle requires that an accounting