How does the presence of the lone pair affect the geometry of the central atom in the following molecule? 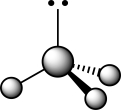
I The lone pair is attracted to the nuclei of the three substituents,creating larger bond angles.
II The lone pair repels the three sets of covalently bonded electrons.
III The lone pair has no bearing whatsoever on the VSEPR geometry at the central atom.
IVThe bond angles are smaller than a traditional tetrahedral bond angle due to lone pair repulsion.
Definitions:
Barbiturates
A class of drugs previously used for anxiety, insomnia, and seizure control, but now less common due to the risk of dependency and overdose.
Knowledge-Management Systems
Specialized systems designed to capture, organize, and distribute knowledge within an organization to improve efficiency, creativity, and innovation.
Audience Perspective
The viewpoint or angle from which members of an audience understand, interpret, or perceive presented information.
Document Revision
The act of reviewing and modifying a document to correct errors, update information, or improve clarity and overall quality.
Q7: Tropyllium bromide is an ionic organic compound
Q12: Assume that the S & B partnership
Q14: The following mechanism step is _. <img
Q26: Draw a detailed mechanism for the following
Q34: For the following E2 reaction,what would happen
Q48: During 2010,Carpenter invested $75,000 and DiAngelo invested
Q72: When an equity security is sold,the sale
Q83: When a partner is added to a
Q136: A company reported net income of $275,000,net
Q161: A company owns $400,000 of 7% bonds