When applying VSEPR theory to determine the geometry about a central atom,it is important to count the total number of bonded and nonbonded electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many bonded electron groups must be considered for each of these central atoms? 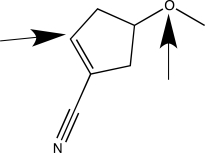
Definitions:
Learned
Acquired knowledge or skill through education, experience, or being taught.
Misinformation Effect
A phenomenon where a person's recall of episodic memories becomes less accurate due to post-event information being introduced.
Drunken Driving
The act of operating a motor vehicle while impaired by the effects of alcohol.
Newspaper Report
A written or printed account of news events published in a newspaper.
Q21: What is the most likely product of
Q42: A partner can be admitted into a
Q48: Sanuk purchased on credit £20,000 worth of
Q49: The following compounds can react via an
Q59: Consider a mixture that is 80% (+)enantiomer
Q70: What is the IUPAC name for the
Q102: Identify the three types of classifications for
Q108: Savan Co.purchased 14,000 shares of Briton Corporation's
Q154: A company had net income of $2,660,000,net
Q177: To prepare consolidated financial statements when a