How does the presence of the lone pair affect the geometry of the central atom in the following molecule? 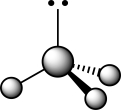
I The lone pair is attracted to the nuclei of the three substituents,creating larger bond angles.
II The lone pair repels the three sets of covalently bonded electrons.
III The lone pair has no bearing whatsoever on the VSEPR geometry at the central atom.
IVThe bond angles are smaller than a traditional tetrahedral bond angle due to lone pair repulsion.
Definitions:
Population Bias
A type of bias that occurs when the sample chosen for a study or survey is not representative of the overall population, leading to skewed or invalid results.
Evidence-based Assessment
The application of research and empirical evidence to evaluate the validity and reliability of assessments or measurements.
Survey
A research method used for collecting data from a predefined group of respondents to gain information and insights on various topics.
High School Students
Adolescents who are enrolled in grades 9 through 12 in the educational system, typically aged between 14 and 18 years.
Q2: What are the approximate H<font face="symbol"></font>C<font face="symbol"></font>H
Q22: What is the most likely product of
Q24: Which is the least stable cyclohexane conformation?<br>A)Chair<br>B)Half-chair<br>C)Twist-boat<br>D)Boat<br>E)Crown
Q40: Which of the following is a coordination
Q43: From left to right,identify the hybridization of
Q43: How many fluorine atoms are in equatorial
Q48: For the following set of reaction conditions,use
Q79: Short-term investments are readily convertible to cash
Q93: A company uses a sales journal,purchases
Q107: Elaine Valero is a limited partner in