Assume that the following molecule is planar.Use the Frost method to explain whether it is aromatic,antiaromatic,or nonaromatic.In your Frost diagram,fill in all π electrons,and label the HOMO and the LUMO. 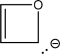
Definitions:
Formaldehyde
A colorless, strong-smelling gas used in making building materials and many household products, known chemically as CH2O.
Lewis Structure
A diagram that represents the distribution of valence electrons around atoms in a molecule, indicating bonds and lone pairs.
Lewis Structure
A graphical representation of the bonding between atoms in a molecule, showing valence electrons as dots.
Double Bond
A chemical bond in which two pairs of electrons are shared between two atoms, usually making the compound more reactive than a single bond.
Q4: Show how the following compound could be
Q12: In the following reaction,identify all electron-rich and
Q13: What is the product of the following
Q15: Supply two possible starting materials for the
Q15: Draw the detailed mechanism and the product
Q16: Two students need to carry out the
Q28: Which of the following wavelengths corresponds to
Q35: Draw the product of the following reaction.Pay
Q35: Which of the following could be the
Q36: Give the product of the following reaction.