Which species is represented by the following distribution of π electrons in the molecular orbitals and energy levels diagram? 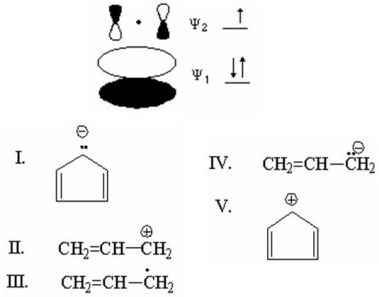
Definitions:
Covalent
A type of chemical bond where atoms share pairs of electrons.
Hydrogen Chloride
A gaseous compound made of hydrogen and chlorine; when dissolved in water, it forms hydrochloric acid.
Lewis Structure
A graphical representation showing the arrangement of valence electrons around atoms within a molecule, indicating bonds and lone pairs.
Lone Pair
A lone pair refers to a pair of valence electrons that are not shared with another atom and are located in the outer shell of an atom, contributing to the molecule's shape.
Q19: Provide the structure of the major organic
Q29: Which is the correct order of decreasing
Q30: Which of the following terms best describes
Q49: If (S)-glyceraldehyde has a specific rotation of
Q69: Which of the following is chiral?<br>A)cis-1-bromo-3-chlorocyclobutane<br>B)trans-1-bromo-3-chlorocyclobutane<br>C)cis-1,4-dimethylcyclohexane<br>D)cis-1,3-dimethylcyclohexane<br>E)trans-1,3-dimethylcyclohexane
Q85: Provide the structure of the major organic
Q120: A mixture of equal amounts of two
Q123: Provide the systematic name of the compound
Q160: In the acetate ion (CH<sub>3</sub>CO<sub>2</sub><sup>-</sup>),which of the
Q172: The Diels-Alder reaction is a concerted reaction;