How does an acid catalyst speed up the reaction below? 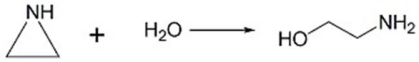
Definitions:
Coase Theorem
A principle stating that if trade in an externality is possible and there are no transaction costs, bargaining will lead to an efficient outcome regardless of the initial allocation of property rights.
Externalities
Monetary consequences or side effects that touch upon third parties not directly engaged, with potential for both positive or negative outcomes.
Private Bargaining
Private bargaining refers to the negotiation process between individuals or private entities without external intervention, aimed at reaching mutual agreements or resolving disputes.
Laissez-faire Style
A leadership or management style characterized by minimal direct supervision, allowing employees significant autonomy in their day-to-day operations.
Q13: Which of the following amino acids contain
Q28: Give the final product of an amino
Q30: What organic compounds are formed when linoleic
Q65: Propose a mechanism for the vitamin KH<sub>2</sub>-catalyzed
Q72: Provide the structure of thymine; How is
Q74: Identify the first step in the mechanism
Q85: The major organic product in the following
Q91: Draw glutamate.
Q109: The lone pair in pyridine is present
Q124: What is the major alkene formed in