Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen.
4Al(s) + 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 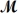
= 26.98 g/mol) and 117.65 g of oxygen ( 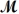
= 32.00 g/mol) is allowed to react.What mass of aluminum oxide ( 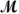
= 101.96 g/mol) can be formed?
Definitions:
Source Document
Original records that contain important details of business transactions, serving as evidence for accounting purposes.
Biased or Unbiased
A characteristic of information or opinion that shows prejudice for or against something (biased) or represents fairness and impartiality (unbiased).
Primary Research
Collection of original data that is done firsthand by the researcher for a specific research purpose or goal.
Q4: Describe in brief how electronegativity values can
Q22: For each of the following elements,indicate whether
Q25: A compound of bromine and fluorine is
Q30: To complete the first setup on a
Q50: Tetrasulfur dinitride decomposes explosively when heated.What is
Q51: An electron in the n = 6
Q64: A 1.30-L sample of argon gas is
Q171: _ describes the flow of goods,services,and information
Q173: A step cost function is an example
Q182: Which of the following deals with management