Tetraphosphorus hexaoxide ( 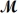
= 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas.
P4(s) + 3O2(g) P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6,what is the percent yield for the reaction?
Definitions:
Table of Contents
A list, usually found at the beginning of a document, that includes the titles or descriptions of the document's contents along with the page numbers on which they are found.
Indirect Approach
A communication strategy where the main point or message is not presented directly at the beginning but is gradually led up to.
Call to Action
A prompt or statement designed to encourage an immediate response or action from the audience.
Executive Summary
A brief overview of a document's key points, designed to give readers a quick preview of its contents.
Q10: A system contracts from an initial
Q12: What is the mass in grams
Q20: For the following equations,<br>a.name the scientist to
Q24: Propane,C<sub>3</sub>H<sub>8</sub>,is commonly provided as a bottled
Q32: Management accounting _.<br>A)focuses on estimating future revenues,costs,and
Q48: Based on electronegativity trends in the periodic
Q51: The compound,NaH<sub>2</sub>PO<sub>4</sub>,is present in many baking powders.What
Q71: Predict the products by completing a balanced
Q86: What is a plausible explanation of a
Q194: Production is the _.<br>A)generation of,and experimentation with,ideas