What is the percent yield for the reaction
PCl3(g) + Cl2(g) PCl5(g)
If 119.3 g of PCl5 ( 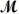
= 208.2 g/mol) are formed when 61.3 g of Cl2 ( 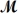
= 70.91 g/mol) react with excess PCl3?
Definitions:
Maximize Profit
The process by which a company determines the optimal output and pricing to achieve the highest possible financial gain.
Total Revenue
The entire amount of revenue produced through the selling of products or services prior to deducting any costs.
Economic Profits
Profits exceeding the opportunity costs of all resources employed, indicating an above-normal return.
Monopolist
An entity or firm that is the exclusive provider of a product or service in a market, enabling it to control prices and market conditions.
Q6: Bud N.Chemist must determine the density of
Q11: Write down the full electron configuration for
Q14: The Starship Enterprise is caught in a
Q35: Hydrogenation of double and triple bonds
Q44: Generally a coefficient of determination (r<sup>2</sup>)of 0.30
Q56: The lattice energy of MgCl<sub>2</sub> is
Q56: Select the gas with the largest root-mean-square
Q67: What is the density of carbon dioxide
Q77: Which one of the following is
Q82: Consider the balanced equation:<br>Al<sub>2</sub>S<sub>3</sub>(s)+ 6H<sub>2</sub>O(l)