How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 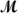
= 84.02 g/mol) ?
HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
Definitions:
Hallucinations
Perceptions or sensations experienced without the presence of external stimuli, typically auditory or visual.
Social Withdrawal
The tendency to avoid social interactions and seek solitude, which can be a symptom of psychological distress or disorders.
Emotional Expression
The way individuals convey their feelings, whether through facial expressions, gestures, or words.
Demonological Model
An explanation for phenomena, including disease and mental illness, based on the belief in evil spirits or demons.
Q7: A system that does no work
Q39: Discuss the cost-benefit approach guideline management accountants
Q39: For which of the following elements (in
Q45: Calculate the molar mass of tetraphosphorus decaoxide,P<sub>4</sub>O<sub>10</sub>,a
Q47: Potassium permanganate is a strong oxidizer that
Q51: Select the correct set of products
Q53: Select the classification for the following
Q58: Which of the following is a covalent
Q66: If a managerial accountant suspected his or
Q92: The best-designed strategies are valuable,whether or not