Magnesium reacts with iron(III) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron.
3Mg(s) + 2FeCl3(s) 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 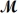
= 24.31 g/mol) and 175 g of iron(III) chloride ( 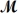
= 162.2 g/mol) is allowed to react.What mass of magnesium chloride = 95.21 g/mol) is formed?
Definitions:
Stream
A small, natural body of flowing fresh water, leading from one to another or into a larger body of water.
Worldwide Distribution
The global spread or availability of a product, service, or phenomenon across the world.
Altitude
The height of an object or point in relation to sea level or ground level.
Latitude
A geographic coordinate that specifies the north-south position of a point on the Earth's surface.
Q6: Consider the set of isoelectronic atoms and
Q12: In the cumulative average-time learning model,cumulative average
Q15: a.Calculate the momentum of a photon of
Q18: Which of the following contains ionic bonding?<br>A)CO<br>B)SrF<sub>2</sub><br>C)Al<br>D)OCl<sub>2</sub><br>E)HCl
Q43: What is the formula for lithium nitrite?<br>A)LiNO<sub>2</sub><br>B)Li<sub>2</sub>NO<sub>2</sub><br>C)LiNO<sub>3</sub><br>D)Li<sub>2</sub>NO<sub>3</sub><br>E)LiNO<sub>4
Q47: Calculate the density in g/L of gaseous
Q52: Which one of the following equations
Q52: Consider the following adjectives used to describe
Q82: Which of the following is an example
Q89: Hydroxylamine hydrochloride is a powerful reducing agent