How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 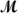
= 84.02 g/mol) ?
HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
Definitions:
HR Supply
The available pool of candidates that an organization's human resources department can draw upon to fill positions.
Demand Requirements
The specific needs or conditions that must be met for a service, product, or project, often detailing quantities, specifications, and timing.
External Environmental Supply
The provision of resources or inputs to an organization from its external environment, which can include materials, information, or energy.
Organizational Demand
The requirements or needs of an organization that dictate its operational, personnel, and resource requirements.
Q4: Write down the oxidation number of the
Q21: Iron (III)chloride hexahydrate is used as a
Q36: Which of the following statements concerning an
Q44: Select the classification for the following
Q46: The standard heat (enthalpy)of formation of graphite
Q47: The ripening of fruit,once picked,is an example
Q54: When an alkali metal combines with a
Q54: Lithium oxide is an effective absorber
Q59: Elements in which the outermost electron has
Q63: Calcium fluoride,CaF<sub>2</sub>,is a source of fluorine and