Use Hess's Law to calculate the enthalpy change for the reaction
WO3(s) + 3H2(g) W(s) + 3H2O(g)
From the following data: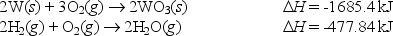
Definitions:
Certificate Of Deposit
A Certificate of Deposit (CD) is a financial product offered by banks and credit unions that provides an interest rate premium in exchange for the customer agreeing to leave a lump-sum deposit untouched for a predetermined period of time.
Multiple Endorsements
Refers to the practice in banking and finance where a document, such as a check, is signed over to another party multiple times.
Liable
Being legally responsible for something, typically referring to obligations in law.
Primary Liability
The direct and immediate responsibility to fulfill an obligation or repay a debt, without necessarily having recourse against others for reimbursement.
Q26: Which of the following compounds is ionic?<br>A)PF<sub>3</sub><br>B)CS<sub>2</sub><br>C)HCl<br>D)SO<sub>2</sub><br>E)MgCl<sub>2</sub>
Q27: Which of the following terms refers to
Q35: The average distance from Earth to
Q41: The coordination number of sodium and chloride
Q42: Select the gas with the highest average
Q46: Nitrogen will behave most like an ideal
Q49: Which one of the following statements about
Q53: The reaction<br>2NaOH(aq)+ H<sub>2</sub>SO<sub>4</sub>(aq) <span class="ql-formula"
Q54: A single water molecule can participate in
Q64: The energy gap between the conduction band