Use the following data to calculate the standard heat (enthalpy) of formation, H°f ,of manganese(IV) oxide,MnO2 (s) .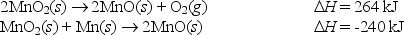
Definitions:
Hands-on Leadership
A leadership style characterized by the leader's direct involvement in the work and decision-making processes of the team.
Managerial Level
Refers to the specific layer or tier in an organization's hierarchy that is responsible for directing and overseeing employees and operations.
Top-level Executive
A high-ranking official within an organization, typically part of the executive team or C-suite, responsible for making strategic decisions that affect the entire organization.
Organization Culture
The shared values, beliefs, and practices that shape the social and psychological environment of a business or organization.
Q12: What is the mass in grams
Q13: A Snickers® candy bar contains 280
Q18: Calculate the mass in grams of
Q27: In an endothermic reaction,in going from the
Q34: Which of the following should have the
Q39: In which one of the following structures
Q47: Valence bond theory predicts that tin will
Q51: An electron in the n = 6
Q55: Aluminum oxide (used as an adsorbent
Q57: The difference in energies between the 1s