Multiple Choice
Use Hess's Law to calculate the enthalpy change for the reaction
WO3(s) + 3H2(g) W(s) + 3H2O(g)
From the following data: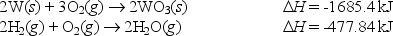
Definitions:
Related Questions
Q7: In which one of the following is
Q20: A 0.89% (w/v)sodium chloride solution is referred
Q31: Potassium fluoride is used for frosting glass.Calculate
Q40: Select the best Lewis structure for ClCN.
Q43: According to molecular orbital (MO)theory,the twelve outermost
Q52: Which one of the following equations
Q59: Select the precipitate that forms when aqueous
Q75: Which of the following is a weak
Q77: The substance,CaSe,is used in materials which are
Q83: The substance,KClO<sub>3</sub>,is a strong oxidizer used in