Calculate the Enthalpy Change for the Reaction
NO(g)+ O(g) NO2(g)
from the Following Data:
A)-551
Calculate the enthalpy change for the reaction
NO(g) + O(g) NO2(g)
From the following data: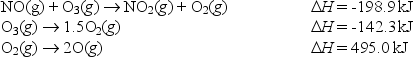
Definitions:
Ice-Water Soaked
Describes items or materials that have been submerged or drenched in a mixture of ice and water, often used for cooling or therapeutic purposes.
Urinary Catheter
A flexible tube used to empty the bladder and collect urine in a drainage bag, aiding individuals who have difficulty urinating naturally.
Electrolyte Levels
The concentration of minerals in the body that are essential for various bodily functions.
Auscultate
The act of listening to the sounds made by internal organs, typically with a stethoscope, as a part of medical examination.
Q4: Describe in brief how electronegativity values can
Q10: The average distance between the Earth
Q11: Briefly explain the relationship between hypothesis and
Q12: Which of the following elements has the
Q15: Which one of the following statements about
Q35: Calculate the number of moles in 17.8
Q50: Tetrasulfur dinitride decomposes explosively when heated.What is
Q54: Platinum,which is widely used as a
Q55: _ includes providing financial information for reports
Q61: The S.I.base unit of mass is<br>A)mg<br>B)g<br>C)kg<br>D)metric ton<br>E)lb