Oxygen difluoride is an unstable molecule that reacts readily with water.Calculate the bond energy of the O-F bond using the standard enthalpy of reaction and the bond energy data provided.
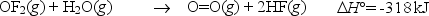

Definitions:
Introverts
Individuals who prefer minimally stimulating environments and tend to recharge by spending time alone.
Contradiction
A situation or statement in which two or more things are opposed to each other or are mutually exclusive.
Radar Machine
A device that uses electromagnetic waves to detect the position, velocity, and other characteristics of distant objects, commonly used in navigation and weather forecasting.
Microwave Oven
An electric oven that uses microwaves to heat or cook food, allowing for quick preparation times.
Q4: Continuous spectra are characteristic of molecules in
Q23: Which of the following oxides will be
Q23: The surface tension of water is lowered
Q31: Line spectra are characteristic of atoms in
Q32: Identify the element of Period 2 which
Q37: According to VSEPR theory,a molecule with the
Q43: Sulfur dioxide reacts with chlorine to
Q46: Nitrogen will behave most like an ideal
Q65: Consider the phase diagram shown below. <img
Q69: Which one of the following relationships