In the following Lewis structure for ClO3F,chlorine has a formal charge of ____ and an oxidation number of ____. 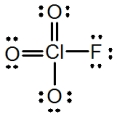
Definitions:
Indirect Method
A way of calculating cash flows from operating activities in the cash flow statement by starting with net income and adjusting for changes in balance sheet accounts.
Depreciation Expenses
The systematic allocation of the cost of a tangible asset over its useful life.
Accounts Receivable
Money owed to a business by its customers for goods or services that have been delivered but not yet paid for.
Accounts Payable
Liabilities of a firm that are due to be paid to creditors within a short period of time, usually within a year.
Q9: Natural gas,or methane,is an important fuel.Combustion
Q11: For the solid forms of the following
Q22: Use the Rydberg equation to calculate the
Q37: Name each of the following functional groups:
Q40: Select the best Lewis structure for ClCN.
Q43: An important step in the synthesis
Q48: A first-order reaction has a half-life
Q68: Hexachlorophene is used as a disinfectant in
Q83: Select the correct Lewis structure for NOCl,a
Q86: A characteristic reaction of alkanes is addition.