Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 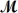
= 86.18 g/mol) at 49.6°C.P°hexane = 400.0 torr at 49.6°C.
Definitions:
Prescriptive Analytics
The set of analytical techniques that yield a best course of action.
Maximize
To increase or make as large or great as possible, often used in the context of mathematical optimization.
Minimize
To reduce to the smallest possible amount or degree.
Constraints
Restrictions or limitations on the variables of a problem which define the conditions or boundaries within which a solution must be found.
Q21: Which of the following ions will be
Q21: Examine the following phase diagram and determine
Q22: A molecule with the formula AX<sub>3</sub> uses
Q34: Calculate the molarity of a solution prepared
Q42: In a single atom,what is the maximum
Q44: According to valence bond theory,which of the
Q48: A container was charged with hydrogen,nitrogen,and ammonia
Q50: The maximum number of phases of a
Q52: Copper(II)bromide is used as a wood preservative.What
Q68: Nitric oxide and bromine were allowed