Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 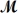
= 74.55 g/mol) in 100.0 g of water.Assume ideal behavior for the solution;Kf = 1.86°C/m.
Definitions:
Efficient Markets Hypothesis
The Efficient Markets Hypothesis (EMH) posits that all known information is already reflected in stock prices, making it impossible to consistently achieve higher returns through stock market predictions.
Ex-Dividend Date
The specific date on which a stock is set to go "ex-dividend," meaning that shareholders of record as of that date will be entitled to receive the declared dividend.
Declaration Date
The specific date on which a company announces its next dividend payment, detailing the amount and when it will be paid.
Date of Record
The specified date when a company determines the owners of its stock to be eligible for a forthcoming dividend or corporate action.
Q2: Which of the following statements concerning
Q8: Magnesium hydroxide is used in several
Q13: Thionyl chloride is used as an oxidizing
Q14: Hexagonal close packing of identical atoms occurs
Q15: Arrange aluminum,nitrogen,phosphorus and indium in order of
Q16: Which of the following aqueous liquids will
Q29: In molecular orbital theory,combination of two atomic
Q36: In applying molecular orbital theory,the bond order
Q45: Which of the following aqueous liquids will
Q93: Select the correct name for the following