Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 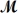
= 74.55 g/mol) in 100.0 g of water.Assume ideal behavior for the solution;Kf = 1.86°C/m.
Definitions:
Tiebout Hypothesis
A theory suggesting that people will migrate to local jurisdictions that best satisfy their preferences for public goods and services, reflecting a form of market efficiency in local government.
Public Good
A good that is non-excludable and non-rivalrous, meaning it can be used by many people simultaneously without depletion.
Free-rider Problem
A situation in which some individuals consume more than their fair share of a public resource, or shoulder less of the cost of its production, benefiting from goods or services without paying for them.
Nonexcludable Problem
An issue characteristic of public goods, where it is not possible to prevent individuals who do not pay from consuming a good or service.
Q10: Arrhenius bases raise the hydroxide ion concentration
Q11: The reaction quotient,Q<sub>c</sub>,for a reaction has a
Q19: Which of the following elements is the
Q40: The kinetics of the decomposition of dinitrogen
Q45: Select the correct electron configuration for Cu
Q55: A study of the decomposition reaction
Q58: Which one of the following statements about
Q61: Which one of the following Lewis structures
Q64: A buffer is prepared by adding 150
Q95: Which compound,if any,will not be optically active?