In the gas phase at 500.°C,cyclopropane reacts to form propene in a first-order reaction.The figure below shows the concentration of cyclopropane plotted versus time.Use the graph to calculate approximate values of
a.the rate of the reaction,600.seconds after the start.
b.the half-life of the reaction,t1/2. 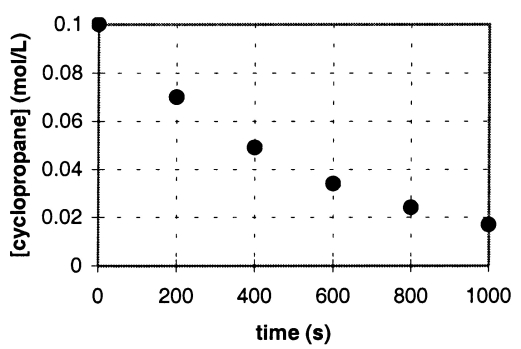
Definitions:
Debt Securities
Financial instruments representing money borrowed that must be repaid, such as bonds, notes, or bills, typically with periodic interest payments.
Stock Securities
Financial instruments that represent ownership shares in a corporation, giving shareholders a claim to part of the company’s assets and earnings.
Consolidated Financial Statements
Combined financial statements of a parent company and its subsidiaries, presenting the financial position and results of operations as a single entity.
Information Overload
A situation where an individual is exposed to more information than they can effectively process or manage.
Q1: A rate constant obeys the Arrhenius
Q6: Cyclobutane decomposes to ethene in a first-order
Q18: In the collision theory of reaction rates,the
Q28: List the three important ways in which
Q43: Identify the organic product when cyclopentanol reacts
Q58: In the gas phase at 500.°C,cyclopropane reacts
Q59: Which relationship or statement best describes
Q64: Write the mass-action expression,Q<sub>c</sub>,for the following chemical
Q64: Oxidation occurs at the cathode of a
Q71: What is the highest possible oxidation state