In the gas phase at 500.°C,cyclopropane reacts to form propene in a first-order reaction.The figure shows the natural logarithm of the concentration of cyclopropane (in mol/L)plotted versus time.
a.Explain how this plot confirms that the reaction is first order.
b.Calculate the first-order rate constant,k.
c.Determine the initial concentration of cyclopropane in this experiment. 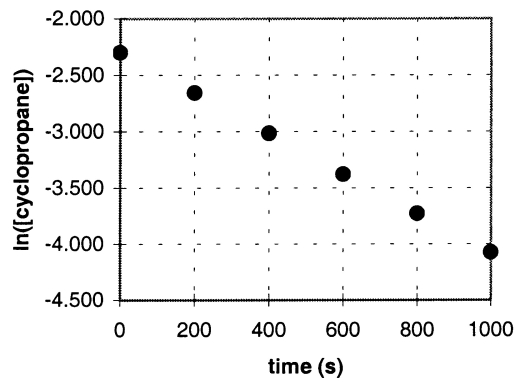
Definitions:
Halo Effect
The cognitive bias where the perception of one positive trait influences the perception of other traits of an individual or entity.
Interpersonal Skills
The abilities an individual possesses to communicate and interact effectively with others, essential for successful personal and professional relationships.
Productivity Standards
Benchmarks or criteria set by organizations to measure the efficiency and performance of their operations and employees.
Perceptual Tendencies
The patterns or biases that influence how individuals perceive information and stimuli from their environment.
Q3: The reaction quotient for a gas phase
Q21: Consider the following mechanism for the
Q24: Which relationship best describes <span
Q28: The equivalence point in a titration is
Q31: Which relationship or statement best describes
Q38: The elements from Groups 1A(1)and 2A(2)are<br>A)strong acids.<br>B)strong
Q45: Which relationship best describes <span
Q47: Consider the equilibrium<br>H<sub>2</sub>(g)+ Br<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg" alt="Consider
Q53: In order to write the correct mass-action
Q67: The equilibrium constant K<sub>c</sub> for the reaction<br>A(g)+