Multiple Choice
Consider the reactions of cadmium with the thiosulfate anion.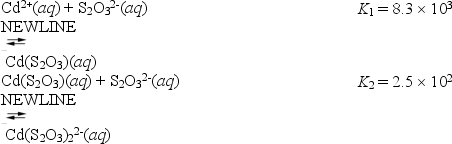
What is the value for the equilibrium constant for the following reaction?
Cd2+(aq) + 2S2O32-(aq) 
Cd(S2O3) 22-(aq)
Definitions:
Related Questions
Q2: Which of the following statements concerning
Q16: A buffer is prepared by adding
Q34: Which of the following ligands could participate
Q35: A certain transition element has the stable
Q40: Buffer solutions with the component concentrations shown
Q49: Name two different classes (types)of hydride.
Q62: Barbiturates are synthetic drugs used as sedatives
Q64: When a catalyst is added to a
Q76: A metal in the simple cubic lattice
Q80: Select the correct type for the following