Calculate S° for the combustion of propane.
C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) 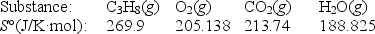
Definitions:
Coining Money
The power to produce and regulate currency, typically exercised by a sovereign government or central authority, ensuring a standardized form of money within its jurisdiction.
Jay's Treaty
A 1795 treaty between the United States and Great Britain that sought to settle outstanding issues from the American Revolutionary War and facilitate ten years of peaceful trade between the nations.
Impressment
The British navy’s practice of using press-gangs to kidnap men in British and colonial ports who were then forced to serve in the British navy.
British Imports
Goods or services brought into a country from the United Kingdom for sale or use.
Q6: All strong acids have weak conjugate bases.
Q9: Identify the missing species in the following
Q13: What will be the effect of adding
Q13: An asset with a large standard deviation
Q14: Buffer solutions with the component concentrations shown
Q15: Which of the following ions could exist
Q32: Which of the following substances has
Q36: Consider the oxides XO<sub>2</sub>,where X is one
Q71: Which of the following is NOT an
Q71: What is the highest possible oxidation state