Calculate G° for the reaction of ammonia with fluorine.
2NH3(g) + 5F2(g) N2F4(g) + 6HF(g) 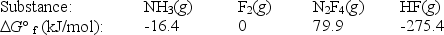
Definitions:
Possibility
The potential for something to happen or exist in the future, often evaluated in risk assessment and project planning.
Identifying Risk
The process of finding, recognizing, and describing risks that could affect the outcome of a project or business operation.
Alternative Method
A different approach or technique used to achieve a goal, often considered when traditional methods are ineffective.
Risk Event
A specific occurrence that can affect the outcome of a project either positively or negatively.
Q1: Identify the products of the reaction between
Q15: The market rewards assuming additional unsystematic risk
Q21: Benzocaine is from a family of chemicals
Q23: A 30.0-kg child receives 2.65
Q41: Write a complete,balanced equation to represent the
Q44: Which of the following statements about bonds
Q48: What is the value of a bond
Q58: The reaction of bromine with an alkene
Q61: For a chemical reaction to be
Q82: All of the following affect the value