Consider the Figure Below Which Shows G° for a Chemical Process Plotted Against Absolute Temperature
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.Which one of the following is an incorrect conclusion,based on the information in the diagram? 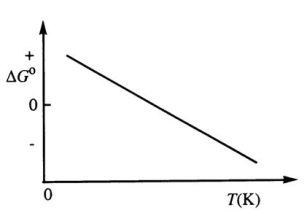
Definitions:
Monopolistic Competition
A market structure characterized by many firms selling products that are similar but not identical, allowing for competition based on quality, price, and marketing.
Industry
A group of companies or organizations that produce similar goods or services.
Purely Competitive
A purely competitive market is one with many sellers and buyers where no single entity has significant control over prices and where products are largely undifferentiated, leading to market equilibrium determined by supply and demand.
Total Profit
The total financial gain made by a business after subtracting all costs and expenses from total revenue.
Q1: The substance NaNO<sub>3</sub> is considered<br>A)a weak Arrhenius
Q2: The chlor-alkali process produces chlorine,Cl<sub>2</sub>(g),in large quantities.What
Q5: Of the 3d transition series of elements,scandium
Q37: The standard deviation of returns on Warchester
Q52: The security market line (SML)intercepts the Y
Q66: The reaction system<br>CS<sub>2</sub>(g)+ 4H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg" alt="The
Q81: At 25°C,the equilibrium constant K<sub>c</sub> for the
Q82: When the following redox equation is
Q88: The capital asset pricing model<br>A)provides a risk-return
Q102: Garvin,Inc.'s bonds have a par value of