Calculate S° for the combustion of propane.
C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) 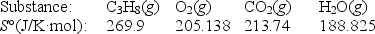
Definitions:
Interest
The charge for the privilege of borrowing money, typically expressed as an annual percentage rate.
Social Mores
Strict norms that control moral and ethical behavior in a culture.
Uniformly
In a consistent or identical manner across a given set of conditions or cases.
Significant
Having a particular meaning, importance, or consequence, often in a specific context.
Q3: The ground state electron configuration of Cr<sup>2+</sup>
Q11: Consider the reaction in the lead-acid
Q18: A 9.52 <span class="ql-formula" data-value="\times"><span
Q25: Huit Industries' common stock has an expected
Q28: In a spontaneous process,the entropy of the
Q34: Investing in foreign stocks is one way
Q54: Carbon monoxide's toxicity is related to its
Q67: Which of the following is true
Q73: A concentration cell is based on
Q79: The Fisher effect can be expressed mathematically