Calculate G° for the reaction of ammonia with fluorine.
2NH3(g) + 5F2(g) N2F4(g) + 6HF(g) 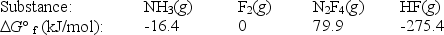
Definitions:
Debenture
An unsecured loan certificate issued by a company, backed only by the general creditworthiness and reputation of the issuer.
Default Risk
The likelihood that a debtor may not fulfill their debt contract obligations.
Yield Maturity
The term generally refers to the date when a bond or other fixed-income security matures, at which point the issuer is obligated to pay the principal amount back to the bondholders.
Asset Pricing
The method of determining the fair value of assets, taking into account various risk factors and expected returns.
Q5: Which relationship or statement best describes
Q6: A 7.85 <span class="ql-formula" data-value="\times"><span
Q24: When inflation rates go up,bond prices go
Q32: Which of the following should have the
Q61: A voltaic cell is prepared using
Q65: For a chemical reaction to be
Q66: All atoms of the first transition series
Q70: A solution is prepared by dissolving 32.0
Q76: The beta of ABC Co.stock is the
Q79: What volume of 0.500 M H<sub>2</sub>SO<sub>4</sub> is