Use the given data at 298 K to calculate G° for the reaction
2Cl2(g) + SO2(g) SOCl2(g) + Cl2O(g) 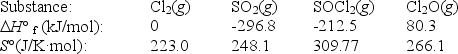
Definitions:
Cows' Milk
The milk produced by dairy cows, commonly consumed by humans and used in a variety of dairy products.
Explicit Instruction
A teaching method that is clear, direct, and structured, often involving step-by-step guidance.
Cultural Evolution
The process by which cultural information and social practices are acquired, preserved, and transmitted across generations, leading to changes in behaviors, beliefs, and norms within a society.
Symbolic
Pertains to symbols or the use of symbols to represent ideas or qualities.
Q6: A battery is considered "dead" when<br>A)Q <
Q8: Butyric acid is responsible for the
Q48: The reaction of methane with water to
Q49: Ammonium chloride is used as an electrolyte
Q54: Carbon monoxide's toxicity is related to its
Q63: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q70: Which one of the following aqueous solutions,when
Q72: Select the nuclide that completes the following
Q73: Lead(II)iodide,PbI<sub>2</sub>,is an ionic compound with a
Q84: The issuance of bonds to raise capital