The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide.
H2O2(l) 
H2O2(g)
Use the following thermodynamic information at 298 K to determine this temperature.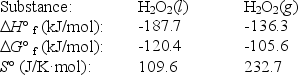
Definitions:
Behavior
Behavior refers to the actions or reactions of an organism or individual, usually in relation to the environment or situation.
Pessimistic
Characterized by a tendency to see the worst aspect of things or believe that the worst will happen; a lack of hope or confidence in the future.
Positive Attitude
A mindset that focuses on the good and positive aspects of any situation.
Frustrated
A feeling of irritation or annoyance due to obstacles, setbacks, or difficulties preventing one from achieving their goals.
Q4: On average,when the overall market changes by
Q21: An isotope with Z > 83,which
Q34: Which of the following ligands could participate
Q48: The reaction of methane with water to
Q50: Which one of the following will give
Q51: Which compound,if any,will be optically active?<br>A)<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg"
Q61: Draw and name all stable molecules with
Q68: When a sky diver free-falls through the
Q90: A formic acid buffer containing 0.50 M
Q94: Identify the two principal products of the