Consider the Figure Below Which Shows G° for a Chemical Process Plotted Against Absolute Temperature
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.Which one of the following is an incorrect conclusion,based on the information in the diagram? 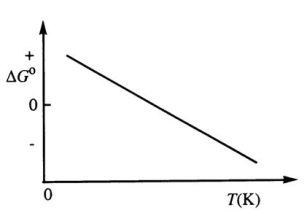
Definitions:
Department of Health and Human Services
A federal executive department of the U.S. government responsible for protecting the health of all Americans and providing essential human services.
Ocular Lens
The lens within the eye that focuses light onto the retina, allowing for clear vision.
Eyepiece
The part of a microscope or telescope that you look through to see the magnified image.
Universal Precautions
Universal Precautions is a set of infection control practices used in healthcare to prevent transmission of diseases that can be acquired by contact with blood, body fluids, non-intact skin, and mucous membranes.
Q15: A 1.25 M solution of the weak
Q16: Helical and sheet-like segments in proteins arise
Q26: A bond with a face value of
Q36: Which of the following is generally used
Q48: The reaction of methane with water to
Q59: The greater the energy of activation,E<sub>a</sub>,the faster
Q64: Oxidation occurs at the cathode of a
Q70: The detailed legal agreement between a bond's
Q74: Write the mass-action expression,Q<sub>c</sub>,for the following chemical
Q102: Garvin,Inc.'s bonds have a par value of